U.S. Food and Drug Administration (FDA) Approves FoundationOne®Liquid CDx as a Companion Diagnostic for Pfizer's BRAFTOVI® (encorafenib) in Combination With Cetuximab to Identify Patients With BRAF V600E Alterations in Metastatic Colorectal Cancer

FDA Approves Foundation Medicine's FoundationOne CDx™, the First and Only Comprehensive Genomic Profiling Test for All Solid Tumors Incorporating Multiple Companion Diagnostics | Business Wire
FoundationOne®CDx Technical Information Foundation Medicine, Inc. 150 Second Street, Cambridge, MA 02141 Phone: 617.418.2200 I
![Workflow for TMB assessment using the FoundationOne CDx assay [63].... | Download Scientific Diagram Workflow for TMB assessment using the FoundationOne CDx assay [63].... | Download Scientific Diagram](https://www.researchgate.net/publication/334081089/figure/fig2/AS:774610417090560@1561692923137/Workflow-for-TMB-assessment-using-the-FoundationOne-CDx-assay-63-ExAC-Exome.png)
Workflow for TMB assessment using the FoundationOne CDx assay [63].... | Download Scientific Diagram
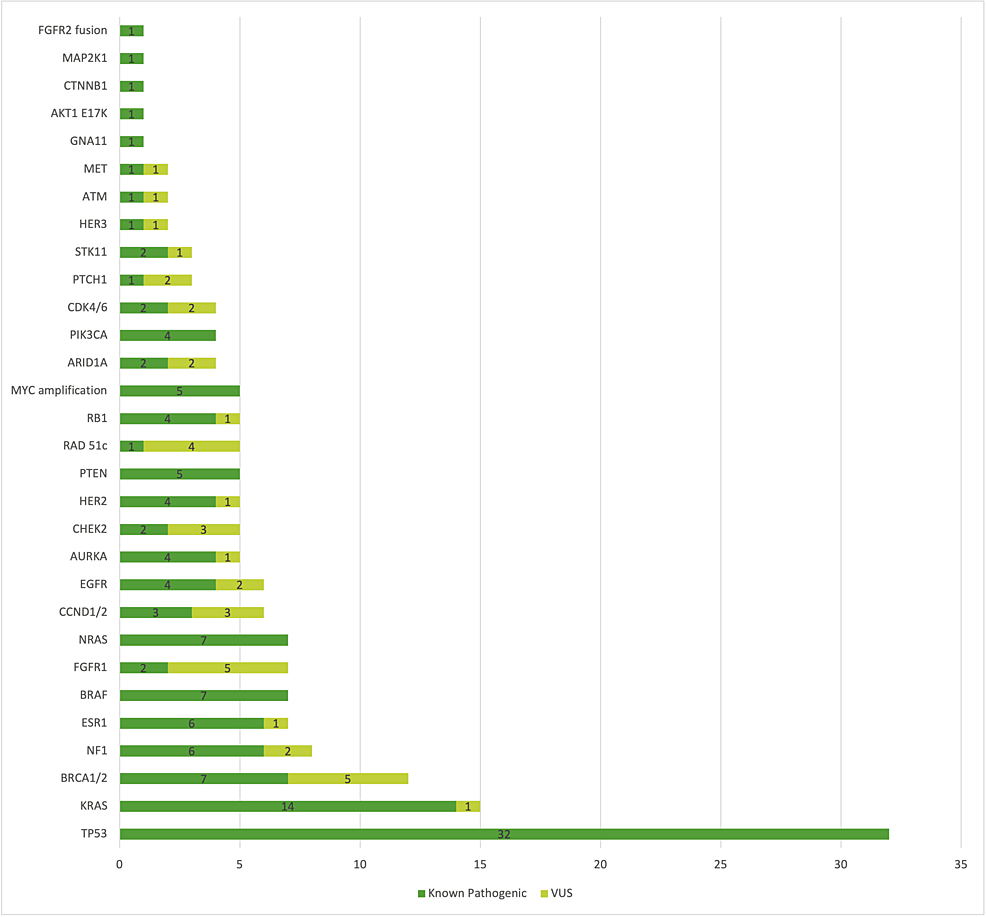
Cureus | Role of Tumor Molecular Profiling With FoundationOne®CDx in Advanced Solid Tumors: A Single-Centre Experience From Romania | Article

![Foundation Medicine review - 7 facts you should know [DEC 2021] Foundation Medicine review - 7 facts you should know [DEC 2021]](https://nebula.org/blog/wp-content/uploads/2021/12/Genomic-findings-1-1024x696.png)