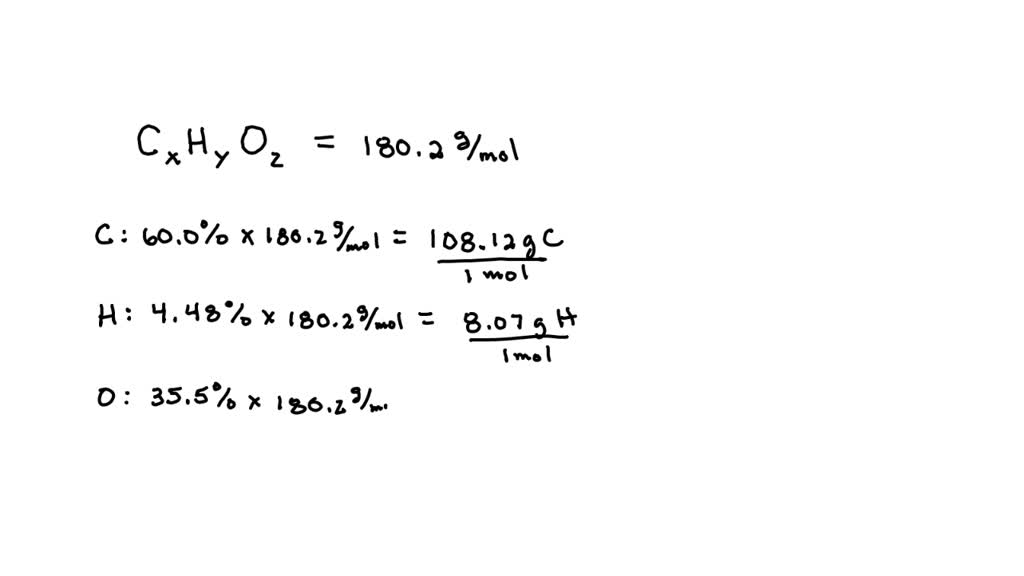If 5.00 g of acetic anhydride (molar mass = 102.1 g/mol) were completely converted to aspirin (molar mass = 180.08), how many grams of aspirin would you expect to make? | Homework.Study.com
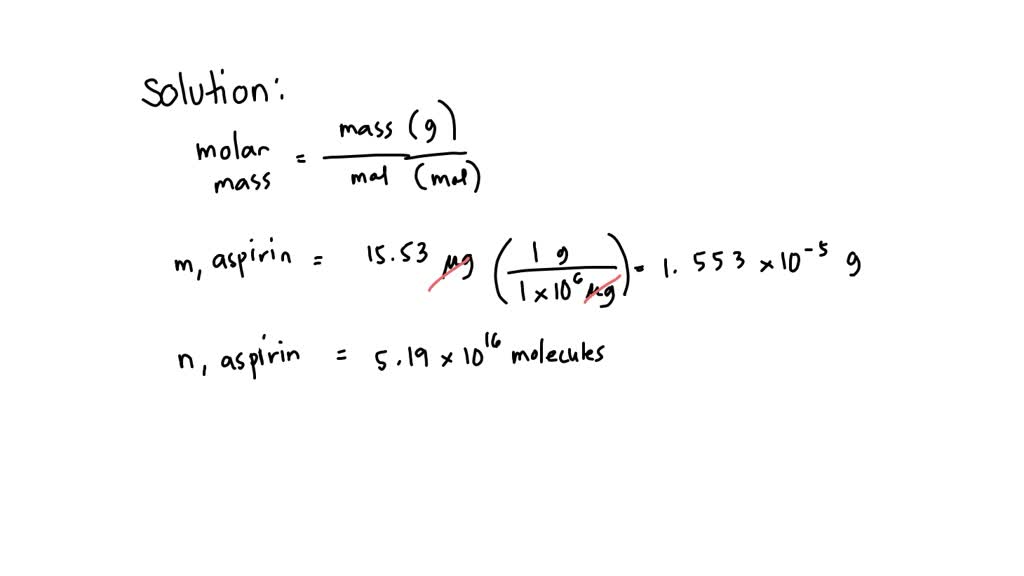
SOLVED: What is the molar mass of aspirin if 5.19 x 10^16 molecules of aspirin weigh 15.53 g? A) 180 g/mol B) 80.6 g/mol C) 133.8 g/mol D) 200 g/mol
SOLVED: What is the molecular weight of aspirin (C9H8O4)? 180.157 amu What is the mass of 0.295 mol of aspirin? How many moles of aspirin are present in 400 mg of aspirin?

Sketch the molecular structure of acetylsalicylic acid and calculate its molar mass. | Homework.Study.com

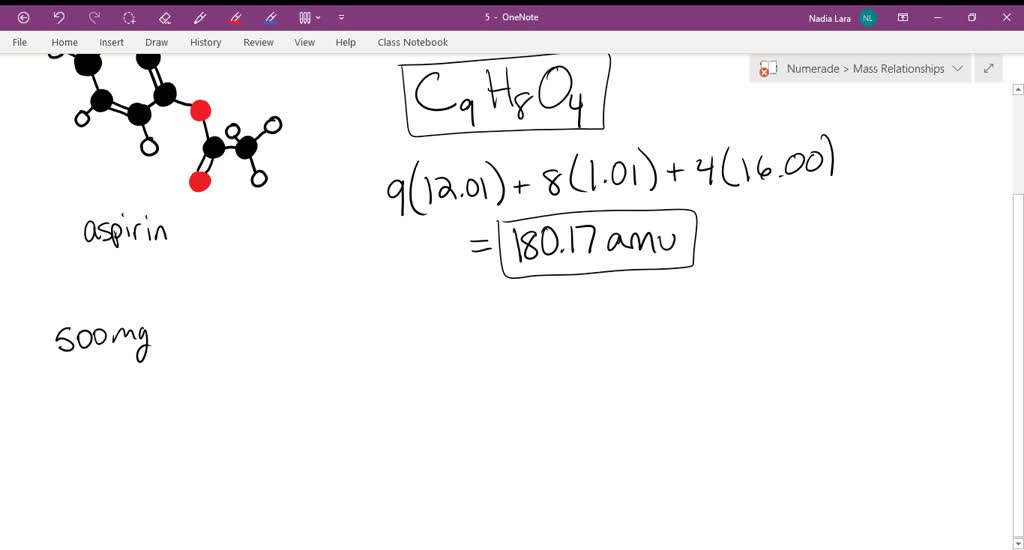
SOLVED: Problem-7 i) Calculate the molar mass (molecular weight) of aspirin: C9H8O4. ii) If each tablet has a mass of 500 mg of aspirin (C9H8O4), calculate the number of moles of aspirin
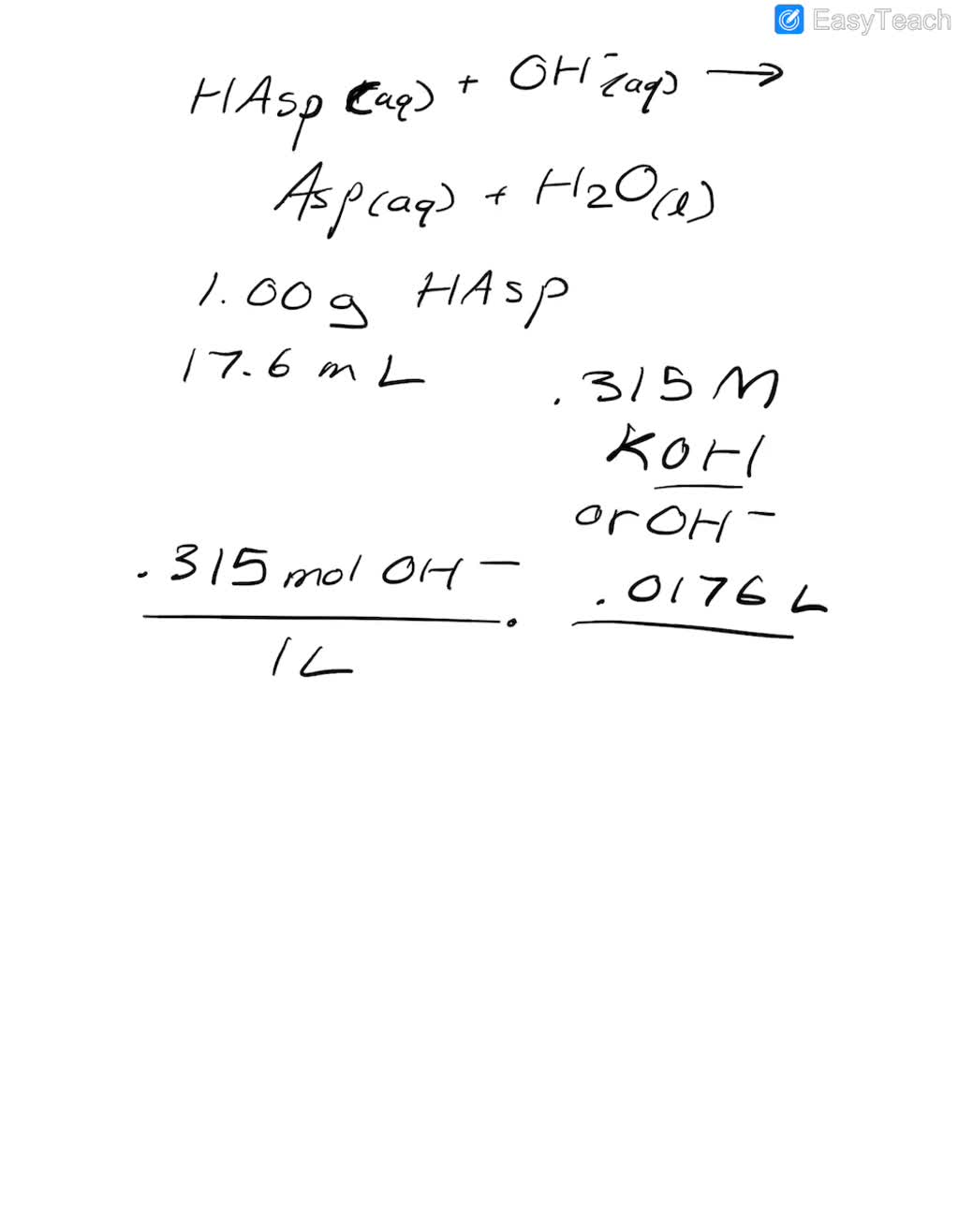
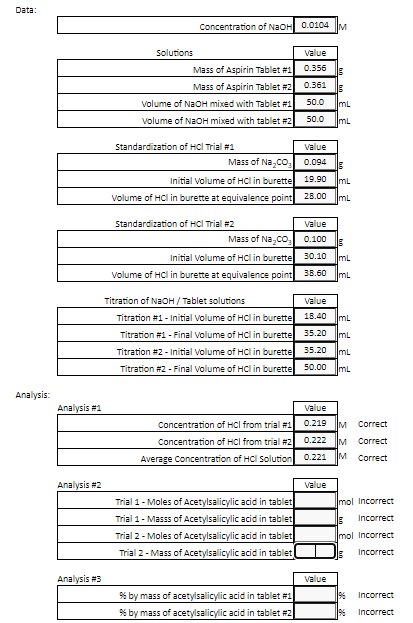
SOLVED: A student tries to determine experimentally the molar mass of aspirin (HAsp). She takes 1.00 g of aspirin, dissolves it in water, and neutralizes it with 17.6 mL of 0.315 M
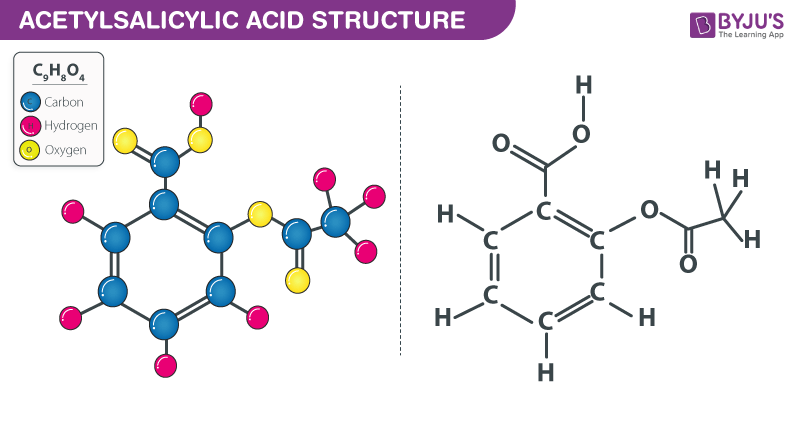
Acetylsalicylic acid (Aspirin ) - C9H8O4 - Formula, Structure, Properties, Preparation, Uses, Health risk and FAQs of Aspirin/ Acetylsalicylic ((C9H8O4)

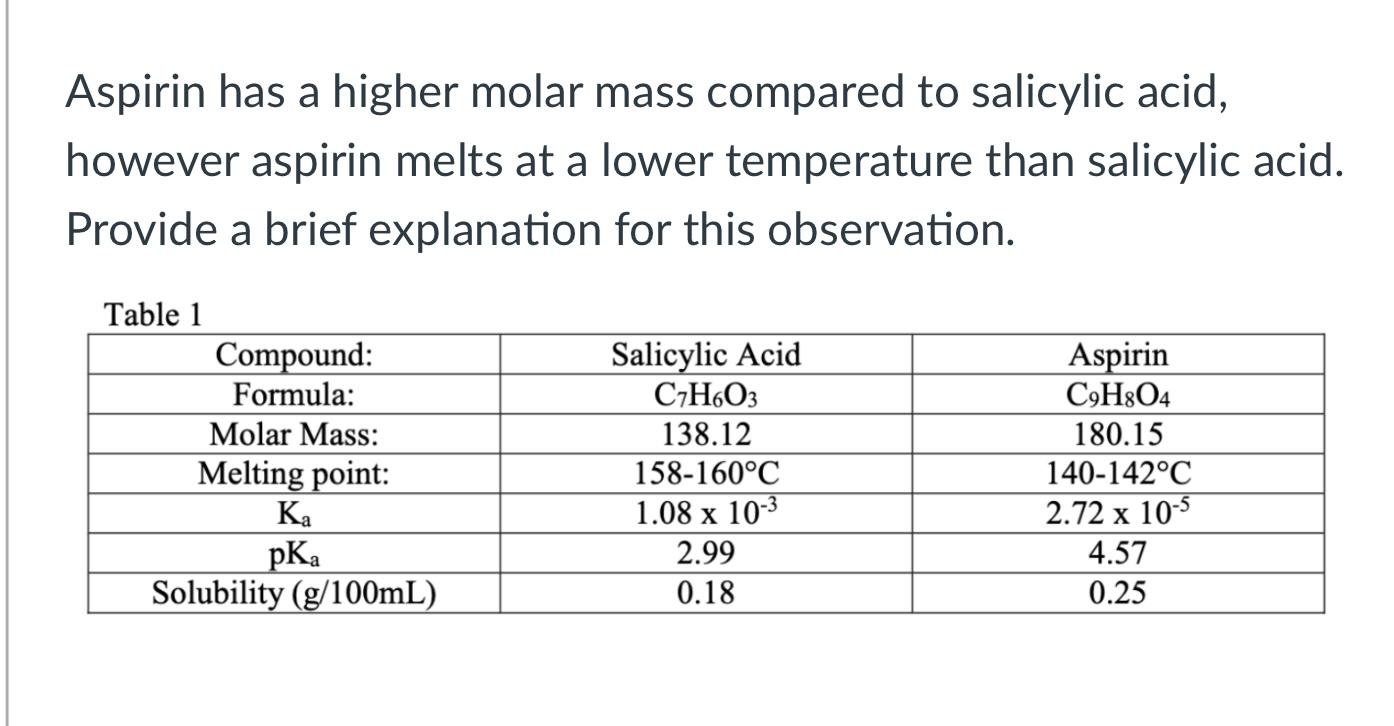
Chemical structure and molecular weight (m.w.) of traditional aspirin,... | Download Scientific Diagram

Calculate the mass percentage of aspirin (C9H8O4) in acetonitrile (CH3CN) when 6.5g of C9H8O4.... - YouTube

Aspirin (acetyl salicyclic acid, molar mass = 180 mol) used as analgesic has pK value of 2. Two tablets of aspirin each weighing 90 mg are dissolved in 100 mL of water.

Calculate the mass percentage of aspirin (C2H804) in acetonitrile (CH:CN) when 6.5 g of CH8O4 is dissolved in 450 g of CH3CN. (Ans: 1.424)

The molecular formula of acetylsalicylic acid aspirin, one of the most commonly used pain reliever - YouTube